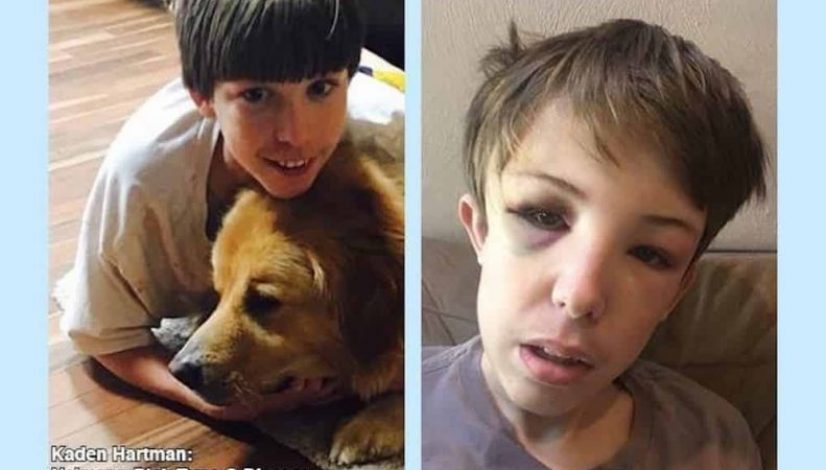
13-Year-Old Denied Life-Saving Treatment Because He Used CBD Oil
Kaden Hartman of Virginia Beach is 13-years-old and lives with Niemann-Pick disease, a condition known colloquially as Child’s Alzheimer’s. There are approximately 500 to 100 cases of diagnosed NPD today, making it extremely rare. Children with Niemann-Pick disease experience rapid physical and mental deterioration. Eventually, they’re faced with seizures and problems with mobility, eating, and communication. According to the National Niemann-Pick Disease Foundation, most children suffering from Child’s Alzheimer’s don’t live to age 20. Kathy Hartman, the mother of this 13-year-old denied life-saving treatment because he used CBD oil, reached out to High Times to explain what happened.
Kaden’s FDA-Approved Treatment
“When there’s nothing else out there to save his life, the FDA will approve an experimental medicine, and he’s been on it for almost three years,” Mrs. Hartman explains.
The drug the FDA approved for Kaden is called Cyclodextrin and has been effectively treating Kaden’s Niemann-Pick disease.
Doctors predicted that Kaden wouldn’t live to age 13, but he’ll be 14 in April.
“It’s definitely working,” says Mrs. Hartman.
The Benefits of CBD Oil

Courtesy of Kathy Hartman
In addition to the experimental Cyclodextrin, Kaden has been taking CBD oil since he started experiencing seizures, a common consequence of Niemann-Pick disease.
“It slows down seizures by, I think, 60 percent,” Mrs. Hartman says of CBD after doing research on the non-psychoactive compound of cannabis on her own.
Watching her son experience 10 to 20 seizures daily, Mrs. Hartman decided to give CBD a shot, under the supervision of Kaden’s primary neurologist.
“It seems to be working great,” Mrs. Hartman told Kaden’s neurologist who closely monitored his CBD use.
With CBD, Kaden avoided the negative side-effects associated with anti-seizure medication.
According to Kaden’s mother, the only potential results of CBD use are slight drowsiness and a better appetite.
Going off CBD, however, has had serious consequences.
After Mrs. Hartman received a letter from the Virginia Commonwealth University Medical Center (VCU), the hospital treating Kaden, stating that Kaden will be taken off the study if he uses CBD, she stopped giving him the herbal supplements.
Off CBD, Kaden experiences many seizures. He has since fractured his skull, concussed himself, and developed two blood clots in his brain.
Not only was this 13-year-old denied life-saving treatment because he used CBD oil, but CBD oil itself was a lifesaving treatment for seizures.
Neurologists who work with Niemann-Pick disease patients recommend CBD Oil
In addition to Kaden’s personal experience with CBD’s benefits, two of his neurologists recommended it.
His primary neurologist of 8 years suggested that Kaden take CBD to cope with his seizures.
Dr. Ralph Northam, Kaden’s former pediatrician who is now the governor of Virginia, wrote Kaden a prescription for cannabidiol.
Though Mrs. Hartman didn’t fill out this prescription at the time, it allowed Kaden access to CBD much more potent than the health store variety he later took.
Dr. Rebecca Caffrey, a friend of the Hartman family, explains, “CBD is the drug of choice for treating seizures in NPC kids.”
She cites another case of two young girls who suffer from Niemann-Pick disease and began taking CBD oil to minimize their seizures.
Not only did CBD help these girls—the daughters of Kathy Hartman’s friend Chris Hempel—but they are allowed to continue their other treatment, which is of the same nature as Kaden’s.
Why the Hospital stopped Kaden’s Treatment
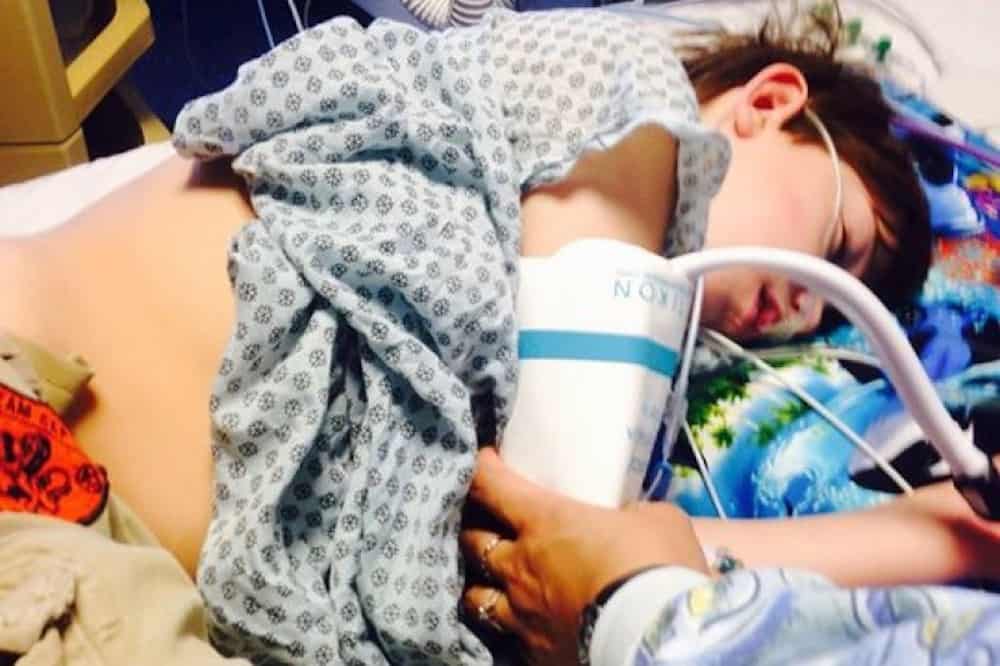
Courtesy of Kathy Hartman
Four days after sending Mrs. Hartman that threatening letter, VCU gave her a call.
“Your son is on CBD oil. He is terminated from the clinical trial. Don’t bother ever showing up again,” Mrs. Hartman says, summing up her conversation.
Confused and infuriated, Mrs. Hartman reached out to VCU repeatedly, to no avail. However, Mrs. Hartman and Kaden showed up to his next appointments.
“I think they tried to trick me,” Mrs. Hartman explains. “I don’t want to break protocol with the FDA because now that would give them reason to terminate. So, I showed up for those [other appointments].”
Though Kaden attended his first two appointments after his trial was terminated, VCU canceled his critical spinal tap appointment held every other Monday.
To date, VCU has amended their statement, though this 13-year-old denied life-saving treatment because he used CBD Oil still cannot access Cyclodextrin.
VCU told Mrs. Hartman that they can get Kaden back on treatment once they have approval from the FDA, the International Review Board (IRB), and the pharmaceutical company.
Mrs. Hartman says that they don’t need any of these approvals due to the nature of Kaden’s treatment and that they shouldn’t be sharing information with the pharmaceutical company in the first place.
“If they’re giving his data to the pharmaceutical company without me agreeing to it, they have a major malpractice suit,” Mrs. Hartman clarifies.
But most importantly, the process of gaining all these unnecessary approvals could take months.
“The FDA only meets once a month. The IRB only meets maybe quarterly or once a month,” Mrs. Hartman worries.
To make matters more difficult, the company that manufactures Cyclodextrin has been bought out twice.
Mrs. Hartman worries that “[Kaden] might never ever get back on this study.”
This could mean that this 13-year-old denied life-saving treatment because he used CBD oil may never again receive the medical care he needs.
CBD doesn’t require FDA approval
Not only do Kaden and others with NPD benefit from CBD, and experts recommend it across the board, but Kaden’s clinical trial never forbade CBD.
The FDA categorizes Kaden’s treatment as an IND: Individual New Drug Investigation.
“He’s not part of a formal clinical trial. He’s a single patient under the compassionate use law,” Mrs. Hartman explains.
This means that Kaden’s treatment is more informal, and they don’t have to get FDA approval for CBD use.
According to Dr. Caffrey, “[Kaden] has received many meds not on the protocol, like steroids for the swelling in his fractured skull and pain meds during his recovery from that injury.”
Not only did doctors give Kaden medication without going through the FDA, but other patients with the same compassionate care drug trials for Niemann-Pick disease routinely take CBD.
Mrs. Hartman believes, “All they have to do is write a two-paragraph letter to the FDA saying FYI we put him on this medicine.”
Kaden’s doctor never even attempted to do so.
Final Hit: 13-year-old denied life-saving treatment because he used CBD Oil
The story of a 13-year-old denied life-saving treatment because he used CBD oil is tragic, unethical and, most likely, illegal.
Every moment that medical professional withholds the only treatment for Niemann-Pick disease, they are endangering Kaden’s life.
“First do no harm. By doing nothing, you’re creating harm,” as Mrs. Hartman told Dr. Syndi Seinfeld of VCU, who is responsible for halting Kaden’s treatment.
Mrs. Hartman looks to the case of her friend Shanon’s son who missed two doses of cyclodextrin and died as a result.
Kaden missed his first dose on January 26th and has missed more since.
Confronted with the injustice of a medical community beholden to pharmaceutical companies and the ignorance of medical professionals, Mrs. Hartman told VCU, “You take him off the study…you just killed my child.”
The post 13-Year-Old Denied Life-Saving Treatment Because He Used CBD Oil appeared first on High Times.